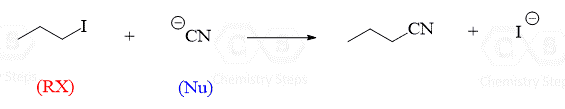
In this post, we will talk about the SN2 mechanism of nucleophilic substitution reactions. As a reminder from the introduction to nucleophilic substitutions, these are reactions where the nucleophile replaces the leaving group:

These reactions are divided into two main types: One, in which the nucleophilic attack and the loss of the leaving group happen at the same time, and the second, in which the loss of the leaving group happens before the nucleophile can attack.
When everything happens simultaneously, it is called a concerted mechanism. This is the SN2 mechanism.
When the processes happen one after the other, it is a stepwise mechanism – the SN1 mechanism.

Let’s zoom in to discuss some details about the SN2 mechanism.
First, what does SN2 mean?
As far as the abbreviation, it stands for Substitution Nucleophilic Bimolecular:

The word “bimolecular” indicates a second-order reaction. To understand this, let’s look at the following reaction:

It is confirmed experimentally that the rate of the reaction depends on the concentration of both the alkyl halide and the nucleophile in first order. Recall that first-order means the rate of the reaction increases linearly with the concentration of the given reactant:

There are two reactants affecting the rate in the first order; therefore, the reaction is second-order overall.
Now, this is a good mathematical description of the rate of the reaction; however, there is more to look at.
For example, what does it mean mechanistically when we say it is a second-order reaction?
A second-order reaction (first order in each in this case) indicates that both reactants collide/participate in the transition state of the reaction:

The dotted lines on the transition state indicate the bonds that are being formed and broken. So, in the transition state, the C-Br bond is partially broken, and, at the same time, the C-O bond is partially formed. Notice that all the bonds are broken and formed in a single step, and this is known as a concerted process – happening simultaneously. We also talk about energy diagrams, including those of SN2 reactions, in the post on Hammond’s postulate.
Reactivity of the Substrate in SN2 Reactions
The most important trend in substitution reactions you need to understand/remember at this point is that less substituted alkyl halides react faster in SN2 reactions. By less substituted, we mean the number of carbons connected to the α and β positions of the carbon connected to the leaving group. You can review the definition of primary, secondary, and tertiary carbons here.
The reason for the higher reactivity of unhindered alkyl halides is the ease of access to the carbon connected to the leaving group. The nucleophile can easier access this carbon and expel the leaving group when there are fewer groups surrounding and thus blocking this route:

One key message you want to remember is to NEVER do an SN2 on a tertiary alkyl halide. The carbon is simply too sterically hindered (crowded) and cannot be attacked by the nucleophile.
The table below shows the relative rate of alkyl halides with different degrees of substitution at their α and β positions:

You will likely not need to remember the numbers, but understanding the reactivity of alkyl halides in substitution reactions will be very helpful at some point when we start talking about determining whether the reaction is SN2, SN1, or perhaps E1, and E2 eliminations need to be considered as well.
The Nucleophile in SN2 reactions
At some point in your Organic 1 class, you will need to determine if the reaction goes by the SN1 or SN2 mechanism.
Remember this – good nucleophiles favor the SN2 mechanism!
Why is it so?
A good nucleophile means it is relatively unstable – reactive, therefore, looking forward to reacting with an electrophilic center.
This is contrary to weak nucleophiles such as water and alcohols. These are stable molecules and happy to stay as they are – they are not desperate to react with an alkyl halide. Thus, weak nucleophiles favor the SN1 mechanism.
The list of common good nucleophiles is shown below, and it will be helpful to keep these in mind:

It might be easier said than done, right? How do you remember so many good and poor nucleophiles?
The good news is that, for the most part, the only weak nucleophiles are water and alcohols.
Another general trend to remember is that the conjugate base (deprotonated – negatively charged) of a compound is a better nucleophile than the neutral molecule.
In general, nucleophilicity parallels basicity, meaning a strong base is a good nucleophile and a weak base is a poor nucleophile.
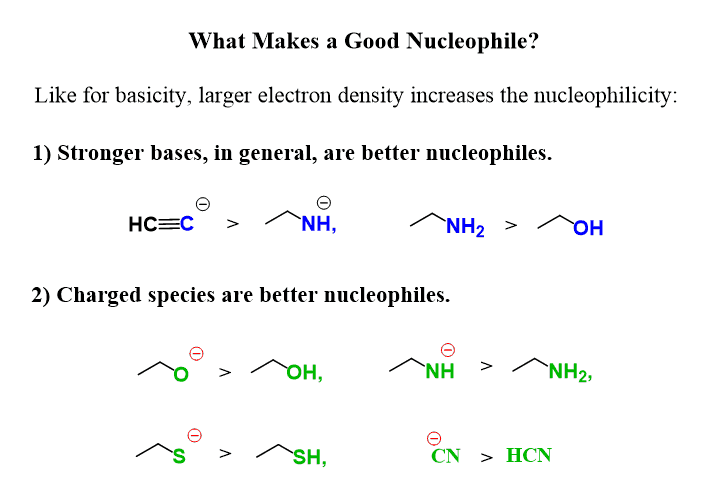
There are, of course, exceptions, mainly because some strong bases tend to be poor nucleophiles as they are too bulky and perform an E2 reaction. This is where we start talking about the competition between SN2 and E2 reactions, which is covered in this article.
More factors to consider when choosing between SN1 and SN2 mechanisms are summarized in this article:
When Is the Mechanism SN1 or SN2?
The Effect of the Leaving Group in SN2 Reactions
The key principle here is that the better the leaving group, the faster the SN2 reaction.

And this is true for any substitution and elimination reaction because the easier it is for the nucleophile to kick out the leaving group, the faster this replacement will occur.
Remember, the bond to the leaving group is partially broken in the transition state. Therefore, its nature does affect the rate of the reaction.
The most common leaving groups in nucleophilic substitution and elimination reactions are shown below:

Notice that the stability of the leaving group has to do with the pKa value of the corresponding acid. The stronger the acid, the more stable (weaker) its conjugate base is, thus the better leaving group it is.
The Effect of Solvent on SN2 Reactions
Nucleophilic substitution reactions occur between polar, and often ionic, molecules. Therefore, they need to be performed in polar solvents so that these species can be solvated.
There are two types of polar solvents: polar protic and polar aprotic.
Polar protic solvents are capable of making hydrogen bonding i.e. they contain a hydrogen connected to an electronegative atom and thus can make intermolecular hydrogen bonding in addition to the dipole-dipole interactions. Usually, either water or alcohol is used for this purpose.
The most common polar protic and aprotic solvents, such as DMF, DMSO, Acetonitrile, HMPA, are listed below:

This is what you need to remember about the solvent in SN1 and SN2 reactions:
Polar aprotic solvents favor SN2, while polar protic solvents favor SN1.
The reason for this is that polar aprotic solvents tend to interact with the counterion of the nucleophile, leaving the nucleophile “naked” and thus more reactive.
For example, when sodium chloride is added to a solution of DMSO, only the sodium ions are solvated, and the Cl– stays as a naked ion, and it is now more reactive:

Remember, a more reactive means a good nucleophile, a nucleophile that wants to react immediately and kick out the leaving group. And this is what the SN2 mechanism is.
On the other hand, when NaCl is dissolved in water, the sodium ion is solvated through a dipole-dipole interaction, and importantly, the Cl– ions are solvated by hydrogen bonding with water molecules, which makes it a weak nucleophile:

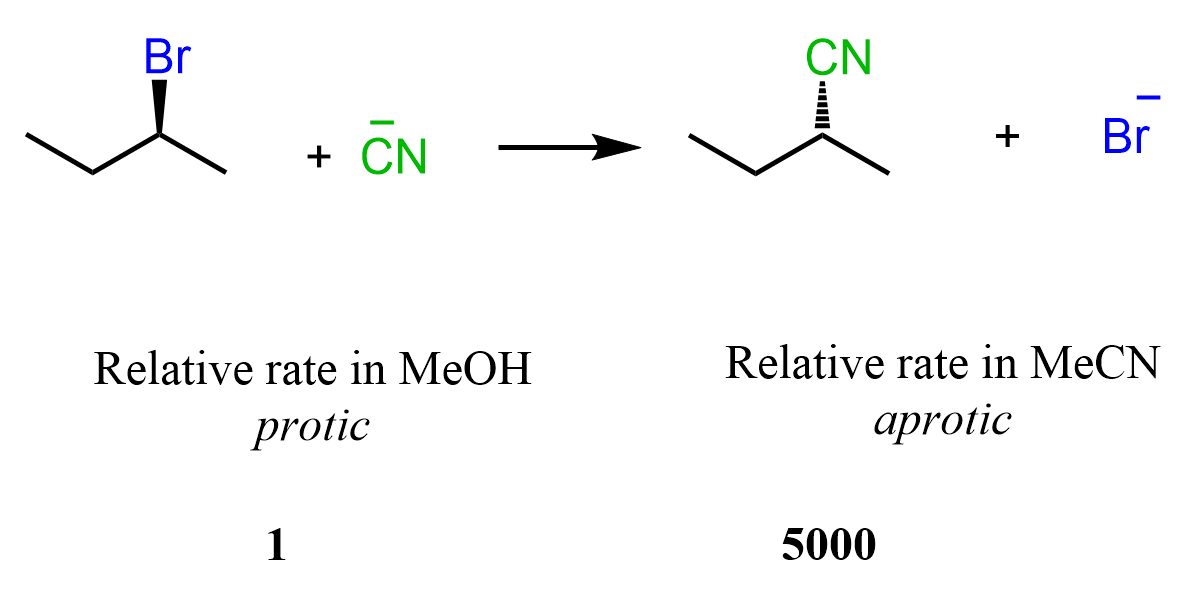
This effect can be illustrated by comparing the relative rate of the SN2 reaction carried out in methanol as a polar protic solvent and acetonitrile as a polar aprotic solvent. You can see the rate increases 5000 times when the reaction is performed in MeCN:
The Effect of Solvent on the Nucleophilicity of Halogens
The trend of favoring the SN2 mechanism in polar aprotic solvents is consistent regardless of what nucleophile is used.
However, the rate enhancement is not the same for every nucleophile. The best example is comparing the rate of SN2 reactions with a fluoride and iodide nucleophiles carried out in methanol and acetonitrile:

The fluoride ion is affected significantly more and reacts millions of times faster when switched to a polar aprotic solvent. It is the most reactive halide ion in SN2 reactions when a polar aprotic solvent is used.
This indicates that the fluoride ion, being the smallest and most electronegative, makes strong hydrogen bonds and is tightly caged by the solvent molecules.
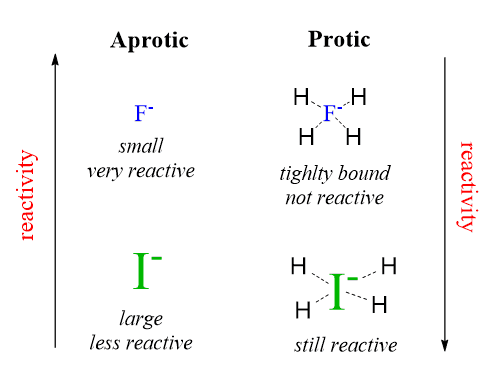
The iodide, on the other hand, is larger and not as electronegative; thus, the effect of a polar protic solvent is not so profound, and it reacts the fastest among all the halogens when the reaction is performed in polar protic solvents.
The Stereochemistry of SN2 Reactions
Primary Alkyl Halides
There is no stereochemistry involved in SN2 reactions of primary substrates since the ɑ-carbon is not chiral:
If there is a chirality center somewhere else in the molecule, do not touch it – it is not part of the reaction:
Secondary Alkyl Halides
If the substrate contains a chirality center, then SN2 reactions proceed with inversion of configuration around this chirality center, also known as Walden inversion. This means if the absolute configuration of the chiral center is S, it becomes R, and vice versa, R becomes S.
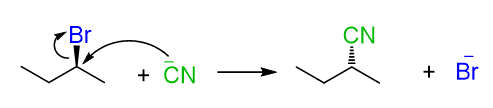
This stereochemical outcome of SN2 reactions is explained by the fact that the nucleophile attacks the leaving group from the opposite side. So, when the leaving group is wedge pointing towards us, the nucleophile attacks from behind the plane, thus appearing in the product as a dash.
The feature of the SN2 mechanism is explained by the molecular orbitals (MO theory) of the nucleophile and the electrophile.
According to this theory, the lone pair(s) of the nucleophile is located in the highest occupied molecular orbital (HOMO) and they flow to the lowest unoccupied molecular orbital (LUMO) of the electrophile. The LUMO is the anti-bonding orbital of the C-Halogen σ bond (σ*).
It is the lowest in energy among all the empty orbitals and therefore the lone pair of the nucleophile is directed there:
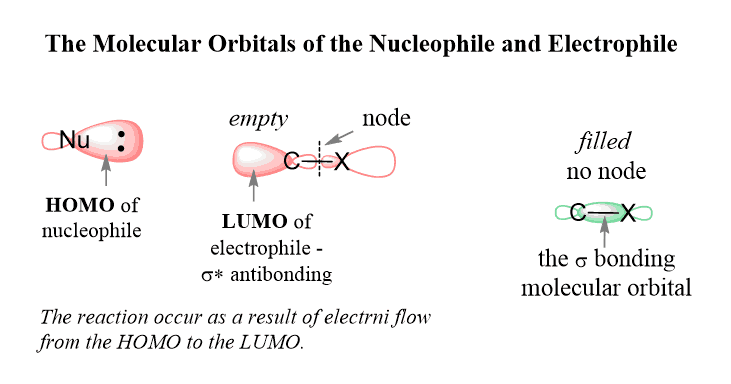
The geometry of the orbitals is such that the antibonding orbital of the C-Br bond is right across the bonding orbital, so the only way of occupying this orbital by the lone pairs of the nucleophile is when it attacks at 180o to the leaving group (back-side attack):

If the leaving group is a dash, then the nucleophile attacks from our side and appears as a wedge in the product:
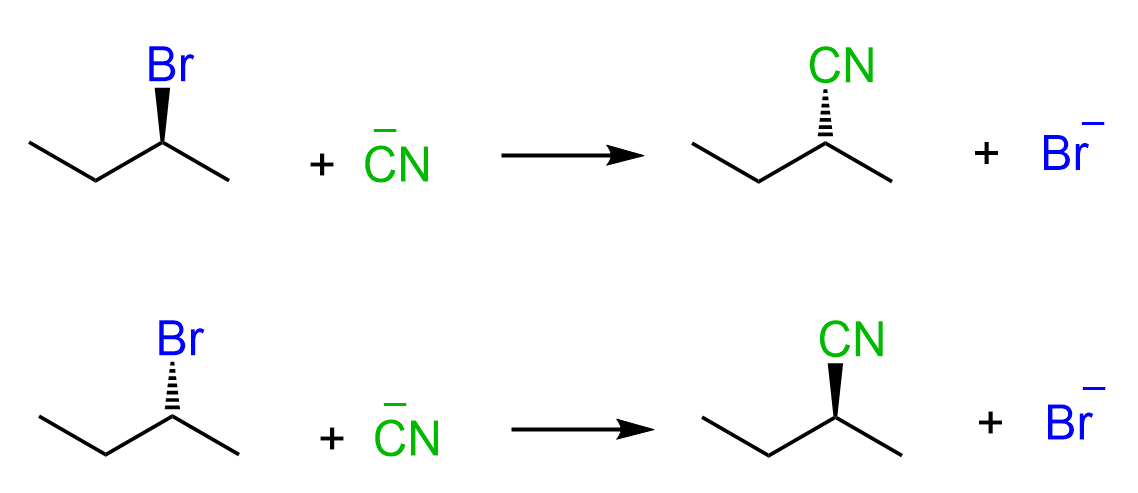
This pattern indicates that the SN2 reaction is stereospecific – the stereochemistry of the product is dependent on the stereochemistry of the starting material.
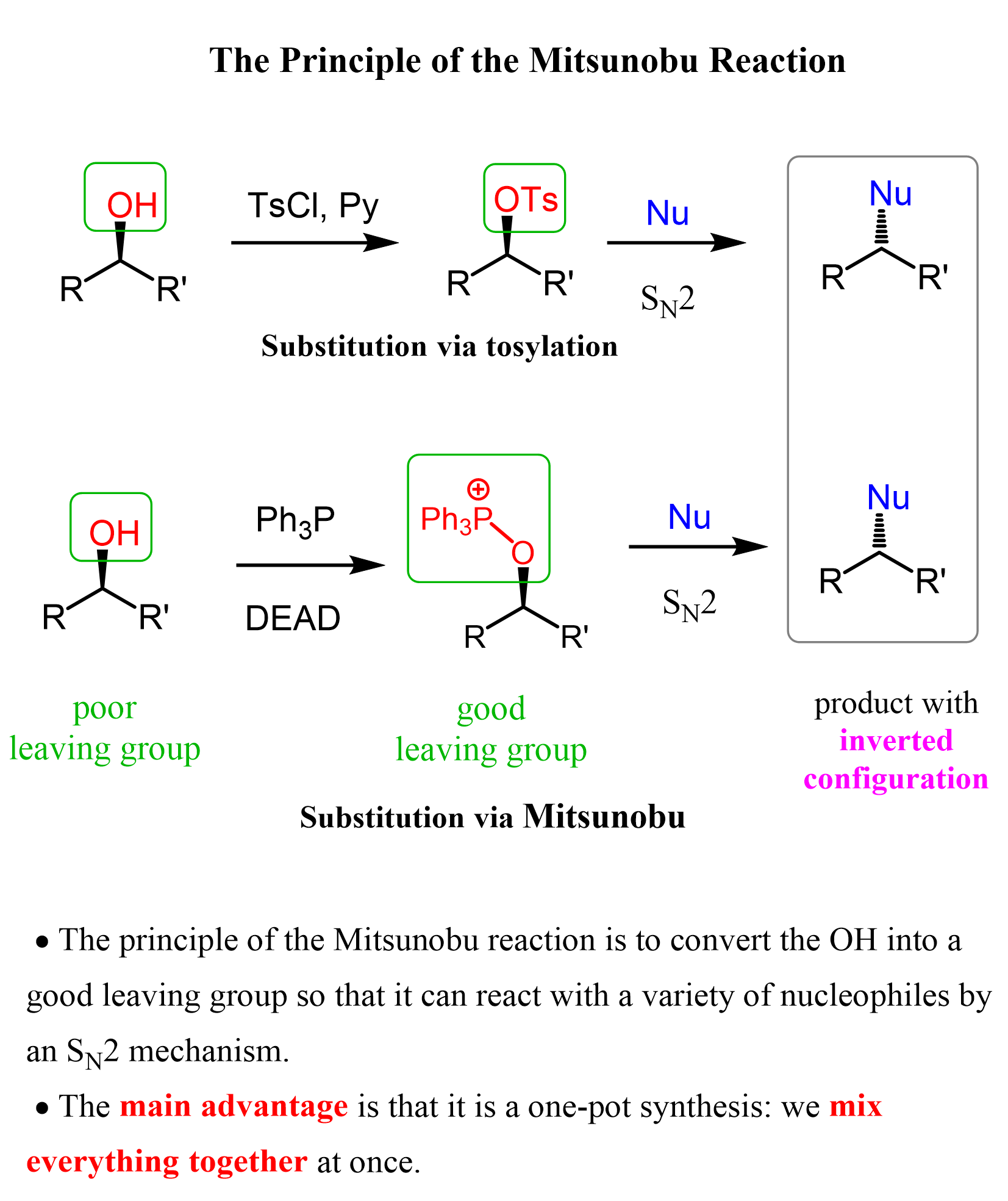
Primary and secondary alcohols can also serve as the electrophile in an SN2 reaction, by converting the OH into a good leaving group, such as a mesylate, tosylate, triflate, or by using the Mitsunobu reaction, which allows this transformation in one-pot synthesis:
SN2 Reactions of Achiral Substrates
The wedge and dash notation is handy for a quick guide; however, you need to keep in mind that it is not an ultimate indicator of the reaction mechanism or the configuration of an atom. Wedge and dash simply depend on the direction we are looking from:
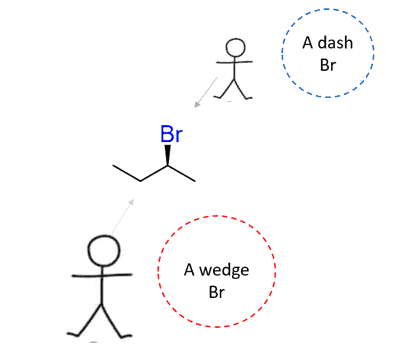
Keep an eye out for tricky questions using the wedge and dash notation.
For example, does the following reaction go by an SN2 or SN1 mechanism?
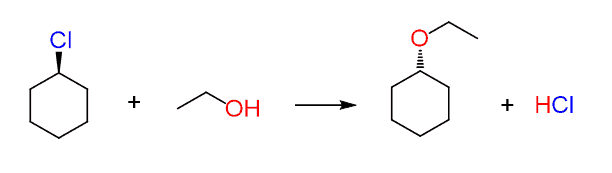
The thing here is that you may look at it and think that since the wedge leaving group is changed into a dash nucleophile, then it must be SN2!?
You should notice, however, that the carbon connected to the leaving group is not chiral since the molecule has a plane of symmetry and the dashed line becomes a wedge by simply flipping the molecule 180o:
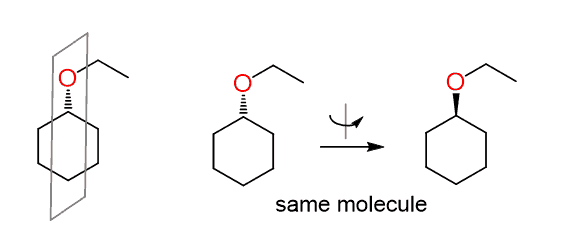
Therefore, sometimes we cannot determine if the reaction is SN2 simply by looking at the wedge and dash lines. In fact, this is a reaction of a secondary alkyl halide with a weak nucleophile and is likely to go by an SN1 mechanism.
Since there is no chirality center, the wedge and dash lines should be normally replaced with plain lines.

Let’s also consider a similar reaction:

The carbon connected to the leaving group is, again, not chiral, and the molecule has a plane of symmetry. However, in this case, we do need to show the wedge and dash lines, and it is not for making it tricky.
The difference with the previous reaction is that there is a methyl group here pointing towards us, and depending on whether the nucleophile appears as a wedge or a dash, we get two different products:
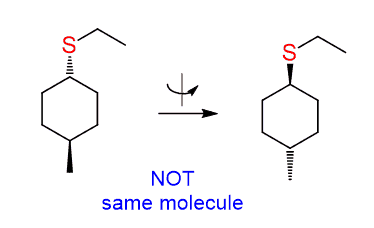
These are cis and trans isomers – a pair of diastereomers and, of course, you can’t do the 180o flip again.
Now, if the reaction goes by an SN2 mechanism, then the nucleophile should appear as a dash – anti to the methyl group since it attacks from the opposite side of the leaving group:
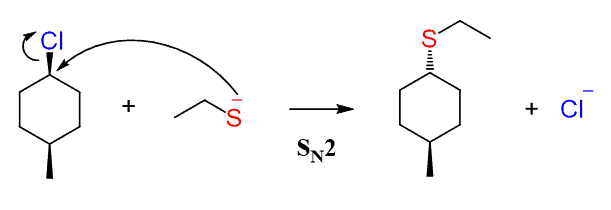
The SN1 reaction would have resulted in a pair of diastereomers since it forms a carbocation intermediate before the nucleophilic attack:
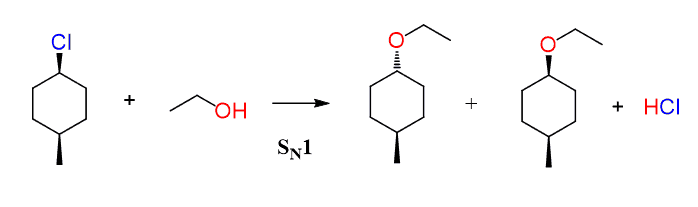
Notice that in any case, the configuration of the methyl group does not change as it is not part of the reaction.
SN2 With Retention of Configuration
As we discussed above, the only sure way of determining whether there was an inversion of configuration or not is by comparing the absolute configuration of the starting material and the product (R and S).
But what if you see a reaction when the absolute configuration of the ɑ-carbon stays the same?
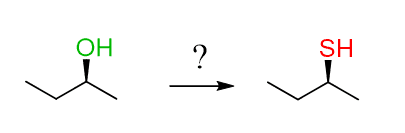
How do you know if this is SN2 or SN1 since we know that the configuration changes from R to S or S to R if it is SN2, and it racemizes in SN1 reactions (check the stereochemistry of SN1 reactions here)?
Well, the SN1 is out since the product is shown as a single enantiomer, meaning that the other enantiomer is not formed.
And the question is, how do you perform an SN2 and keep the configuration the same?
The starting material here is S, and an SN2 reaction would change it to R. However, if we do two SN2 reactions it will go S-R-S:

One approach for achieving this would be converting the OH into a halide, followed by a reaction with sodium sulfide. There are different ways of converting an alcohol into a good leaving group, and you may not be familiar with some of them now:

The method shown above uses the mesylation of alcohols to convert them into a good leaving group, which can then be expelled by the Br– and undergoes another SN2 reaction with the sulfide ion.
This was a three-step synthesis applying double SN2 to retain the absolute configuration, but:
Can an SN2 reaction process occur with retention of configuration?
This is rather an exception that you will likely not need to deal with, but yes, you can have an SN2 reaction where the absolute configuration is not changing.
For example, in the following reaction, the absolute configuration does not change even though it goes through an SN2 mechanism:

The reason for this is that, unlike the leaving group, the nucleophile is not the highest priority on the chiral carbon:

Tertiary Alkyl Halides
Did we say that tertiary substrates do not undergo SN2 reaction?!
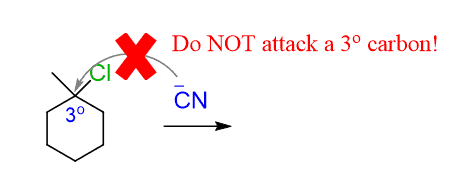
Tertiary alkyl halides undergo SN1 substitution, where the first step is the loss of the leaving group, and only after that, do we have the nucleophilic attack:

There are quite a few important features and reasons you need to know to distinguish the SN2 and SN1 mechanisms, so let’s discuss the latter in a separate article.
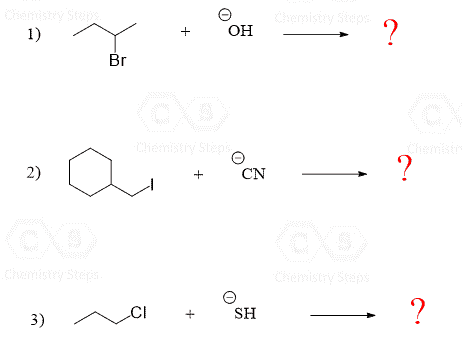
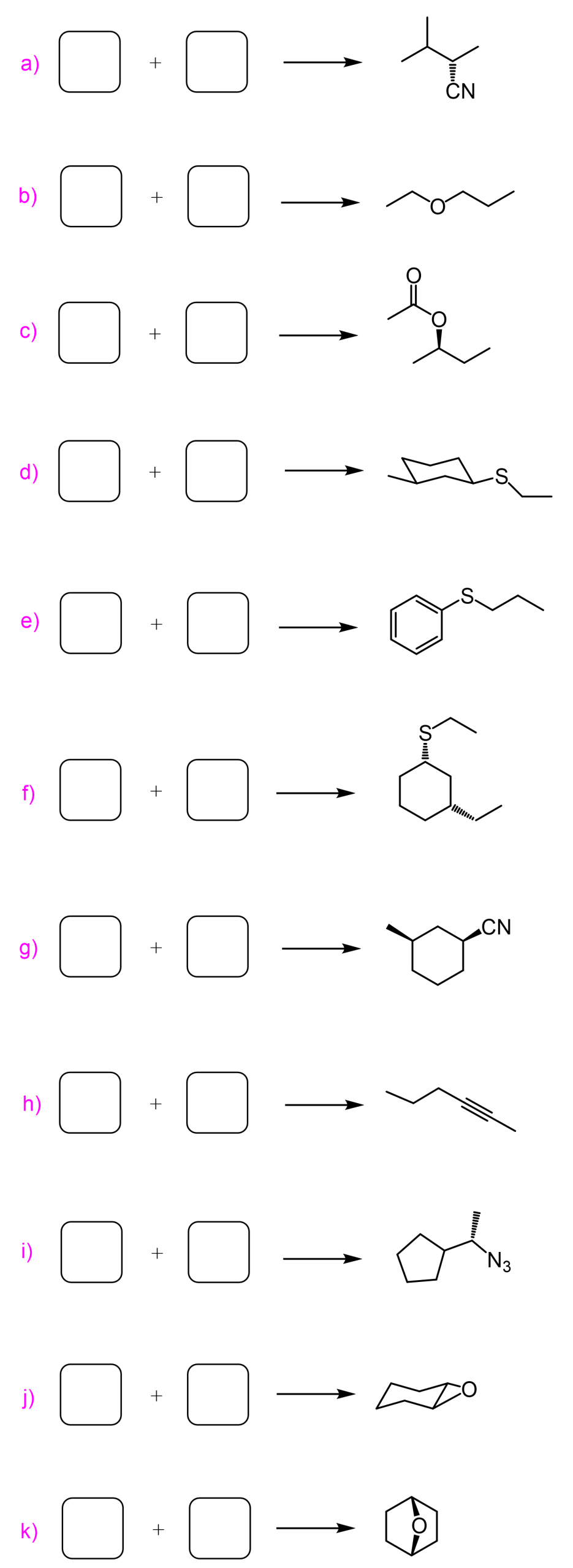
There are some Practice Problems on the SN2 reaction right here
Check out this 65-question, Multiple-Choice Quiz with a 3-hour Video Solution covering Nucleophilic Substitution and Elimination Reactions:
Nucleophilic Substitution and Elimination Practice Quiz








towards the top, it says “the rate only depends on one of the reactants.”
That’s the case for Sn1 but not Sn2 reactions, and while it is minor I think it’s a very confusing typo
That would need to be corrected, but I am not able to find that part. Would you be able to point it out more precisely? Perhaps a copy-paste…
I have a question about the kinetics of the SN2 reaction. So a primary alpha-C reacts faster than a secondary alpha-C. But what if I have a primary alpha-C with a tertiary beta-C? Is the primary one still faster than the secondary or is the steric hinderance too big?
For beta-tertiary substrates, the classical example is the neopentyl group with a halide on the alpha position. Even though it is a primary alkyl halide, the rate of SN2 is way slower (about 10^5 times) compared to regular primary alkyl halides.
And if a nucleophile that is also a strong base (for example HO-, and especially anything bulkier) is used with a substrate containing alkyl groups and a hydrogen in the beta position, for example, isobutyl bromide, the E2 path will likely predominate because of the steric hindrance.
I can’t find exact data on whether a simple secondary substrate will undergo SN2 faster than the neopentyl in the presence of a good nucleophile, but I think it will.
So in presence of NaOCH3 in CH3OH the neopentyl will still react faster than the simple secondary substrate because NaOCH3 is a strong base?
I will need to look it up and see what actual experimental data suggests. The fact that neopentyl halides are 100,000 times slower than primary alkyl halides, makes them essentially unreactive in SN2 reactions. So, as I mentioned above, the secondary should go faster. Are you tested on this? That’s a little dubious of a question if you ask me unless they went over it in class. If you don’t mind sharing, what textbook are you using? It may help me better understand the context this question is being asked in.
The base strength wouldn’t matter here because the neopentyl does not have beta hydrogens and cannot undergo E2 elimination. It can, on the other hand, undergo a substitution via methyl shift rearrangement in an SN1 mechanism, for example, when heated with water.
Ok, from Loudon, Organic Chemistry, isopropyl will do SN2 650 times faster than neopentyl.
Yes so this is basically an exam question and it drives me a bit crazy because I don’t quite know what the right answer is. In my script of the class there is only the basic stuff about SN2.
I study biochemistry in Germany so I will have to translate the question:
Shown are three pairs of potential partners for nucleophilic substitution through NaOCH3. The solvent is CH3OH at low temperature (SN2). Which compound should react faster?
a) CH3-CH2-CH2-CH2Br or CH3-CH2-CH(CH3)Br
b) CH3-CH-CH-CH2Br or CH3-CH2-CH-CHBr
c) C(CH3)3-CH2Br or CH3-CH2-CH2-CH(CH3)Br
Give brief explanations.
(I hope the compounds are understandable)
Yes, tests are sometimes a bit more involving than the lecture material:) I see you have a vinyl bromide too. Be careful with those as they do not form carbocations and are very reluctant in SN2 as well.
Thank you so much for looking that up!
You are best teacher, bro. thanks a lot!
I try 🙂