Choosing Between SN1, SN2, E1, and E2
If you are reading this post, welcome to the world of SN1, SN2, E1, and E2 business! The good news is that you are not alone and that also, we are going to make it as easy as it can get. But remember, this is mastered only by doing lots of practice problems.
So, let’s get started and break this into choosing between two mechanisms rather than four. How?
Well, you first need to remember that SN2 and E2 are bimolecular reactions, meaning that the substrate (alkyl halide) and the base/nucleophile both participate in the rate-determining step. This only happens when the base (if the mechanism is E2) or the nucleophile (if SN2) is strong – very reactive.

If the base/nucleophile is weak, then the mechanism is unimolecular – E1 or SN1.
Let’s summarize this again: if strong – SN2 or E2, if weak – SN1 or E1.

Keeping this in mind, what we see is that essentially you will never have to choose between SN1 vs E2 and SN2 or E1 vs E2 and SN2. That is, if you know the most common strong and weak bases/nucleophiles (see below).
SN1 or E1 – weak base/nucleophile
The weak reactants are mainly going to be the water and alcohols. And choosing between E1 and SN1 is easy – the main factor is the heat. If heat is mentioned, then it is a hint to you that E1 elimination is the main mechanism in that reaction:
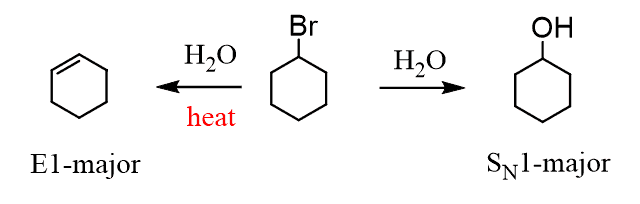
The reason why heat favors elimination over substitution is covered in this post – scroll to the “Why does Heat Favor Elimination?” section.
Notice now that we are already done with the left side of the flowchart, and the only riddle is to choose between SN2 and E2.
SN2 or E2 – strong base/nucleophile
Strong bases take us to the right side (E2 or SN2). Bases can be nucleophilic and non-nucleophilic. Non-nucleophilic means most often it reacts as a base, while nucleophilic base means it can react both as a base and a nucleophile, and this is where the problems start.
For example, ethoxide and tert-butoxide (t-BuOK) ions are (relatively) strong bases. The difference is that the butoxide can only (take it as mostly… I know) act as a base, but the ethoxide reacts as a base and a nucleophile depending on the substrate.
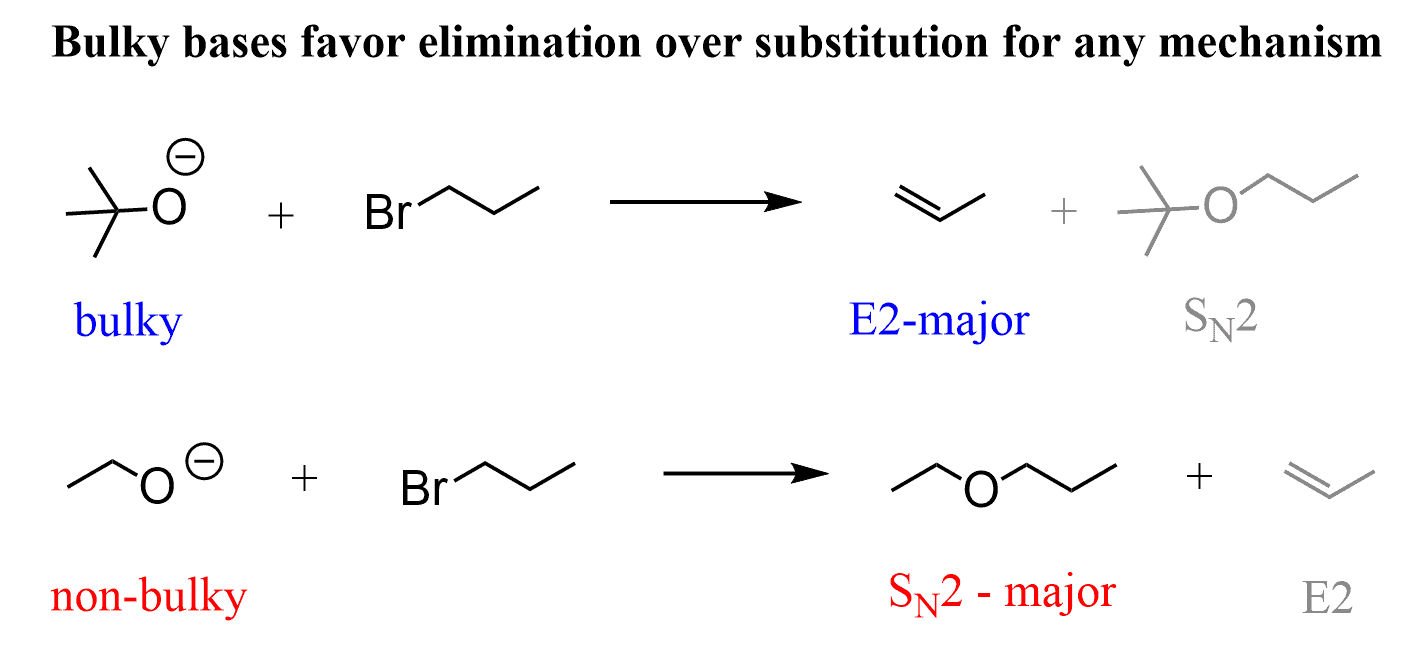
To understand this, remember that steric hindrance favors elimination over substitution for any mechanism.
That is why, if we classify the strong bases as bulky (sterically hindered) and non-sterically hindered, it helps us determine whether it will react as a base or a nucleophile. So, memorize this table with strong bases and nucleophiles:

If you react any of the non-basic nucleophiles with a substrate, you will only get an SN2 product, unless (there are always exceptions, sorry) it is a tertiary substrate, then SN2 is impossible, only SN1 (check this post on choosing between SN1 and SN2).
The basic nucleophiles are carbon, oxygen, or nitrogen-containing (bearing the lone pairs or the negative charge) species. If it is a sterically hindered base, then it can only be E2 – done:
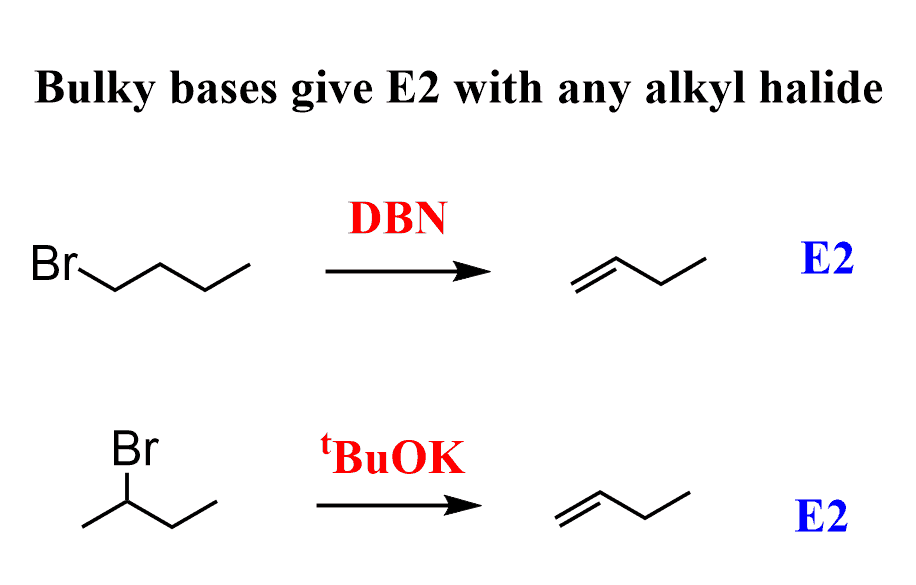
Other common bulky bases are DBU, DIPEA, and LDA.
Notice also that a non-bulky strong base, such as NaOEt, would follow Zaitsev’s rule in an E2 reaction. Now, remember that non-bulky indicates that both SN2 and E2 are possible, and here you need to look at the substrate: if it is primary, you will get SN2, if it is secondary or tertiary, you will get E2 as the major product:

In summary, follow these steps to identify if the mechanism is SN1, SN2, E1, or E2:
1) Determine if the base/Nu is strong or weak
- If strong – SN2 or E2
- If weak – SN1 or E1
2) If it is a strong, bulky base – E2 only. If it is a non-bulky base, look further into the substrate – primary substrates do SN2, secondary and tertiary do E2 as the major mechanism.
The effect of the solvent on nucleophilicity and basicity is another factor (not the main though) to be considered.
Here is a different article about this:
The Role of the Solvent in SN1, SN2, E1, and E2 Reactions
Note: In real life, a mixture of compounds is obtained in most cases. Here we are only talking about general traits and major products. Don’t take words like “never” and “always” literally. There is always an exception, if not more than one.
I hope this was helpful. Check these practice problems-there are plenty. The answers are available to registered members at Is it SN1, SN2, E1, or E2 Mechanism With the Largest Collection of Practice Problems

In addition to this, there is also a multiple-choice quiz on substitution and elimination reactions:
Nucleophilic Substitution and Elimination Practice Quiz
Check Also
- SN1 vs E1 Reactions
- SN2 vs E2 Reactions
- Polar Protic and Polar Aprotic Solvents
- SN1 SN2 E1 or E2 – the Largest Collection of Practice Problems
- Introduction to Alkyl Halides
- Nomenclature of Alkyl Halides
- Substitution and Elimination Reactions
- Nucleophilic Substitution Reactions – An Introduction
- All You Need to Know About the SN2 Reaction Mechanism
The SN2 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems - The Stereochemistry of SN2 Reactions
- Stability of Carbocations
- The SN1 Nucleophilic Substitution Reaction
- Reactions of Alkyl Halides with Water
- The Stereochemistry of the SN1 Reaction Mechanism
- The SN1 Mechanism: Kinetics, Thermodynamics, Curved Arrows, and Stereochemistry with Practice Problems
- Steric Hindrance in SN2 and SN1 Reactions
- Carbocation Rearrangements in SN1 Reactions with Practice Problems
- Ring Expansion Rearrangements
- Ring Contraction Rearrangements
- When Is the Mechanism SN1 or SN2?
- Reactions of Alcohols with HCl, HBr, and HI Acids
- SOCl2 and PBr3 for Conversion of Alcohols to Alkyl Halides
- Alcohols in SN1 and SN2 Reactions
- How to Choose Molecules for Doing SN2 and SN1 Synthesis-Practice Problems
- Exceptions in SN2 and SN1 Reactions
- Nucleophilic Substitution and Elimination Practice Quiz
- Reactions Map of Alkyl Halides
The posts on elimination reactions can be found on the Topics page


Is it more important to look at the substrate or the nucleophile? What if it is a strong nucleophile with a tertiary alkyl halide?
Tertiary substrates cannot do SN2 and the presence of a good nucleophile limits the options to SN1 or E2. If the nucleophile is basic (or the base is nucleophilic), in other words, it is a strong base, then E2 will be the major mechanism. If it is a non-basic nucleophile, listed in the text, then it will be an SN1 reaction.
Why not E1? Because E1 and SN1 are favored by weak bases/nucleophiles.
Why SN1 and not E1? Because SN1 can occur with non-basic, good nucleophiles if the substrate is tertiary.
In most cases, look at the base/nucleophile – if it is strong, you need to choose between SN2 and E2, if weak, it is either SN1 or E1.
When you have to deal with a conflicting combination like the one here, remember the restrictions of SN2 (never on tertiary) and SN1 (never on primary). Again, there are always exceptions such as the allylic and benzylic substrates and primary substrates with a heteroatom that can kick out the leaving group by resonance stabilization.
Elimination
thank you i wonder you for your short and precise elaboration of these four mechanesmes of reaction . i have got these after having read your article.
it is very precise and help full.
Glad to hear that! Thank you.
In the Sn2 or E2 section talking about the strong/bulky base, the photo shows the an extra carbon added to the E2 product for the bulky base. The substrate starts with three carbons whereas there are four in the product.
Indeed, it does. Thanks for spotting that! Fixed!
Wonderful