The E1 is a stepwise, unimolecular – 1st order elimination mechanism:

The first, and the rate-determining step, is the loss of the leaving group, forming a carbocation which is then attacked by the base:

This is similar to the SN1 mechanism and differs only in that, instead of a nucleophilic attack, the water now acts as a base, removing the β-hydrogen:

The E1 and SN1 reactions always compete, and a mixture of substitution and elimination products is obtained:
E1 – A Two-Step Mechanism
Let’s break down the steps of the E1 reaction and characterize them on the energy diagram:

Step 1: Loss of he leaving group.
The energy diagram of the E1 mechanism demonstrates the loss of the leaving group as the slow step with the higher activation energy barrier:

The dotted lines in the transition state indicate a partially broken C-Br bond. The Br being the more electronegative element is partially negatively charged, and the carbon is partially positively charged. Complete ionization of the bond leads to the formation of the carbocation intermediate.
Step 2: Removing a β-hydrogen to form a π bond.
Once the carbocation is formed, it is quickly attacked by the base to remove the β-hydrogen, forming an alkene. Notice the smaller activation energy for this step, indicating a faster reaction:

In the next section, we will discuss the features of SN1 and E1 reactions as well as strategies to favor elimination over substitution.
E1 vs SN1 Mechanism
Let’s mention right from the beginning that bimolecular reactions (E2/SN2) are more useful than unimolecular ones (E1/SN1), and if you need to synthesize an alkene by elimination, it is best to choose a strong base and favor the E2 mechanism.
For example, comparing the E2 and E1 reactions, we can see that one disadvantage of the E1 mechanism is the possibility the carbocation rearrangements:

Just like in the SN1 mechanism, whenever a carbocation is formed, it can undergo a rearrangement.
You can refresh this by going here:
Carbocation Rearrangements in SN1 Reactions
The problem with rearrangements is the formation of a different product that may not be the desired one. For example, the following substrate is a secondary alkyl halide and does not produce the alkene that is expected based on the position of the leaving group and the β-hydrogens:

As shown above, the reason is the rearrangement of the secondary carbocation to the more stable tertiary one, which produces the alkene where the double bond is far away from the leaving group.
Notice that both carbocations have two β-hydrogens, and depending on which one the base removes, two constitutional isomers of the alkene can be formed from each carbocation:

This is the regiochemistry of the E1 reaction, and there is a separate article about it that you can read here.
Remember, on the other hand, that E2 is a one-step mechanism – No carbocations are formed, therefore, no rearrangement can occur.
To demonstrate this, we can run this reaction with a strong base, and the desired alkene is now obtained as the major product:

More details about the comparison of E1 and E2 reactions are covered in this post:
How to favor E1 over SN1
As mentioned earlier, one drawback of the E1 reaction is the ever-standing competition with the SN1 substitution. If a carbocation is formed, it is always going to give a mixture of an alkene with the substitution product:

One factor that favors elimination is the heat. At elevated temperature, heat generally favors elimination over substitution.
Why does Heat Favor Elimination?
This has to do with the greater number of products in elimination reactions. Compare these two reactions:

In the substitution, two reactants result in two products, while elimination produces an extra molecule by reacting with the β-hydrogen.
This means eliminations are entropically favored over substitution reactions. Recall the Gibbs free energy:
ΔG ° = ΔH ° − T ΔS
The entropy factor becomes more significant as we increase the temperature, since a larger T leads to a more negative (favorable) ΔG °.
The temperatures we are referring to here are the room temperature (25 oC) and 50-60 oC when heated to favor elimination.
How to avoid rearrangements in SN1 and E1 Reactions?
What unifies the E1 and SN1 mechanisms is that they are both favored in the presence of a weak base and a weak nucleophile.
The good news is that it is mostly the water and alcohols that are used as a weak base and nucleophile. If a strong base/good nucleophile is used, the reaction goes by bimolecular E2 and SN2 mechanisms:

The focus of this post is on the E1 mechanism; however, if you need it, the competition between E1 and SN1 reactions is covered in a separate post here:
For a broader coverage on the competition between SN1, E1, SN2 and E2 reactions, refer t o this artice: https://www.chemistrysteps.com/sn1-sn2-e1-e2-choose-which-mechanism/
Reactivity of Alkyl Halides in E1 Reactions
Just like in SN1 reactions, more substituted alkyl halides react faster in E1 reactions:

The reason for this trend is the stability of the forming carbocations. The more substituted carbocations are more stable since their formation is the rate-determining step:
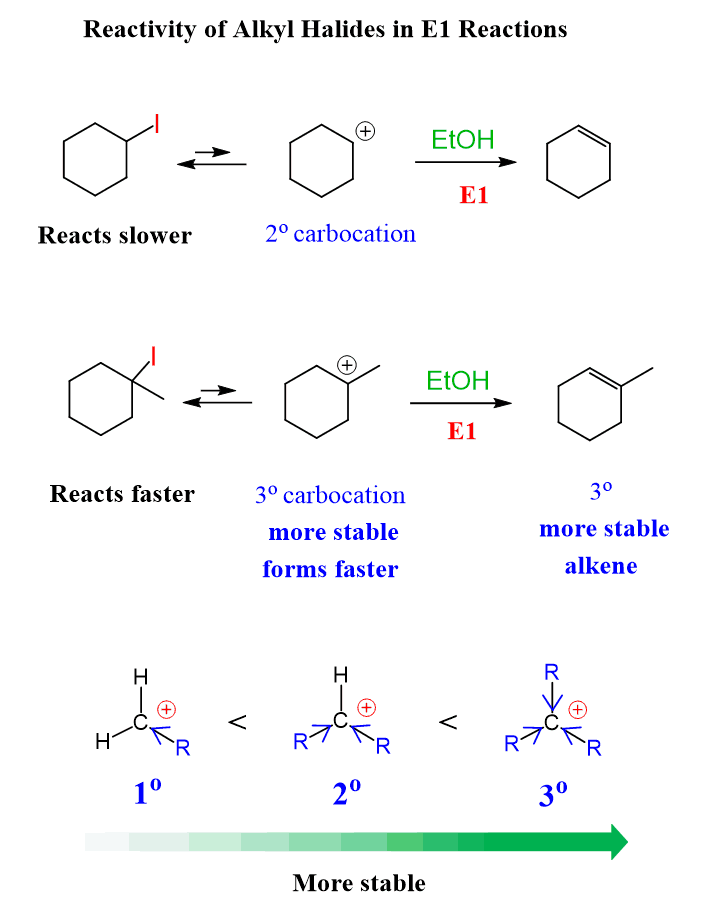
You can read more about the stability of carbocations in this post.




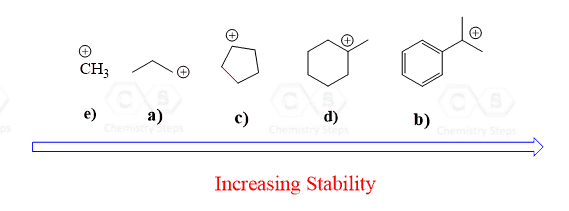





Hi ! In number 5 c), I think the second step is resonnance and not 1,2- hydride shift!
Yes, that is correct. The two carbocations are resonance forms.
You are the super heroes I have understood very well. Congratulations